Research
Nanopore biosensors for precision medicine
Ultra-sensitive, high-throughput biomolecular sensing technologies are transforming basic research and medical diagnostics. For example, recently developed single cell DNA and RNA sequencing methods have been widely used in numerous studies to date, making a huge impact on basic research in life sciences (1). At the forefront of this trend, emerging single-molecule biosensors take a central stage. Specifically, nanopore biosensors are developed as highly accessible and portable devices for extremely accurate studies of genomic, transcriptomics or proteomics information. These studies pave the way towards the development of high precision clinical sensing, for future applications in drug differentiation and personalized medicine.
Current studies in our group are focused on isolation and quantification of specific DNA or RNA biomarkers from blood or plasma samples, using nanopore biosensors. The single-molecule level sensitivity of the sensors highly simplifies the sample preparation process required for sensing, and sharply reduces the amount of clinical sample required. Multiplexing of many different biomarkers (such as single-nucleotide variations) is achieved by specific ligation of DNA barcodes, composed of multiple color tags. The readout of the fluorescent tags is performed by our electro-optical nanopore setup.
©All rights reserved, Meller lab.
DNA translocation through nano-scale pores
Nanopores are essentially single-molecule electrophoretic “gels”. An intense electrical field attracts and threads the electrically-charged DNA strand through a pore embedded in an insulating membrane separating two reservoirs filled with ionic aqueous solutions. The nanopore size is only slightly larger than the biopolymer cross section, hence the molecule unfolds and translocates in a single-file manner through it. The electrical field pulling the DNA through the pore also generates an ion current that can be measured as a function of time. During the passage of the DNA through the pore it partly blocks the ionic current, giving rise to a detectable “resistive pulsing” signals.
Electro-optical sensing in Nanopores
Multi-color, single-molecule fluorescence can substantially expand the sensing capabilities of nanopores by complementing or substituting the resistive pulsing signals. Over the past decade our lab developed nanopore biosensors equipped with simultaneous single-photon detectors. This has allowed us to quantify epi-genetic markers such as methylated cytosines in genomic DNA, resolve DNA barcodes for genotyping applications and distinguish among polypeptides, at the single molecule level.
Dynamic processes in live cells
The translation of cellular mRNA to protein is a tightly controlled process often deregulated in diseases such as cancer. Furthering our understanding of the mRNA structural elements, intracellular proteins and signaling pathways that affect protein expression is crucial for the development of new therapies. To this end, we are developing super-resolution microscopy techniques to image and characterize the process of protein translation initiation in live eukaryotic cells. Our custom-made microscope can acquire images at sub-second temporal resolution, allowing us to dissect a variety of dynamic bimolecular processes in live cells.
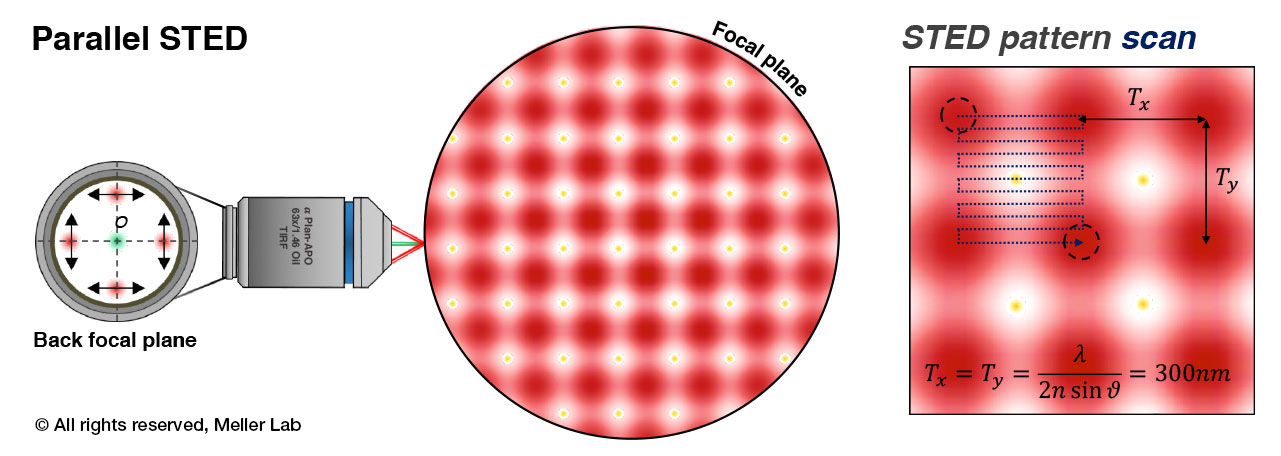
Principles of parallel STED microscopy. An array of STED “cells” is formed by 4-beam interference at the focal plane of the objective lens. This array is quickly scanned with sub-diffraction limit steps to form a super-resolution image with high temporal resolution.
Translation initiation in eukaryotic cells involve the formation of mRNA loops mediated by the interactions of the eukaryotic initiation factors (eIF) and Poly Adenine Binding Proteins (PABP) at the 3’-end of the mRNA. Imaging of the mRNA loop formation in live cells is paramount to understanding the fundamentals of protein synthesis. A fast-scanning parallel STED microscope constructed in our lab enables imaging of these sub-cellular process with sub-second time resolution.
protein localization in Live cells
©All rights reserved, Meller lab.
P53, an important tumor-suppressor gene, has been to shown to preserve genomic stability by recruiting DNA repair proteins upon DNA damage. 53BP1 (p53-binding protein 1) is one such protein that exclusively promotes non-homologous end-joining DNA double strand break (DSB) at damaged chromatin sites. As the dynamics of signaling pathways can be masked when considering a cell population, we have measured the dynamics of 53BP1 in single cells using time lapse fluorescence microscopy. Furthermore, we have exposed MCF10A cells to etoposide, a cytotoxic anticancer drug which prevents re-ligation of DNA strands after unwinding, which invariably results in p53 expression and 53BP1 foci formation several minutes later. As a result, we were able to characterize the time to foci formation, its assembly and subsequent dismantling kinetics.
Single molecule FRET spectroscopy
Single molecule FRET (Förster Resonance Energy Transfer) is a powerful method for measuring inter- and intra-molecular distances at the nanometer scale. We use smFRET to monitor the helicase activity of the eukaryotic initiation factor 4A during RNA unwinding. In the presence of a small accessory factor eIF4H, the helicase unwinds short RNA duplexes upon ATP hydrolysis. But unlike most DNA helices, eIF4A does not translocate along the strand, and instead after unwinding it let’s off one of the strand allowing it to re-zip. This results in a repeated, ATP-driven, unzipping - rezipping kinetics that can be directly observed and quantified by smFRET, by either tagging the RNA molecules with a donor-acceptor pair, or by conjugation of the helices itself (eIF4A) with a FRET pair.
Live videos of the Donor and Acceptor channels of surface immobilized RNA molecules during repeated unwinding-rewinding by eIF4A.
Sm-FRET of immobilized RNA molecules
Surface immobilization of RNA molecules enables long-time visualization of single-molecules in vitro. The molecules are illuminated by Total Internal Reflection (TIR) field allowing imaging of tens of molecules simultaneously. The experiment is performed in a custom-made microfluidic chamber that permits tight control of the buffer conditions and temperature, as well as performing abrupt changes in buffers to observe a time-dependent system response.